Lead Scale Formation and Solubility
Pb oxides
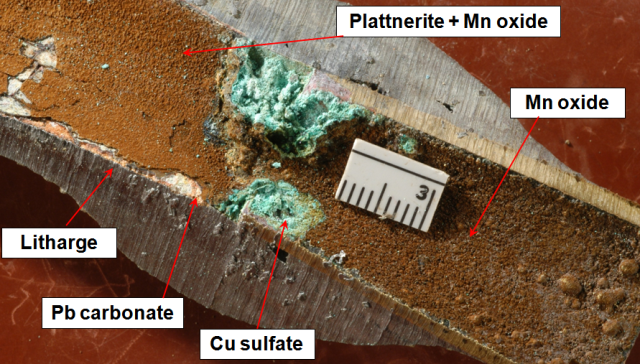
The most common Pb oxide seen in distribution systems is litharge, which is quite soluble, but often occurs as a layer between the metal surface and an overlayer of Pb carbonate. Possibly it forms within the scale by reaction between native lead and the carbonate layer.

Lead service line connected to brass fitting. There are three successive layers on the lead side of the join: litharge (red), lead carbonate (white), and plattnerite (dark reddish brown). On the brass side, the top layer is manganese oxide (chocolate brown).
The Pb4+ oxide, plattnerite is much less soluble than litharge or lead carbonate, but is stable only at very high Eh values (Schock et al., 2001; Schock and Giani, 2004). Such conditions can be achieved in distribution systems with high chlorine residuals throughout and with very low concentrations of organic matter.
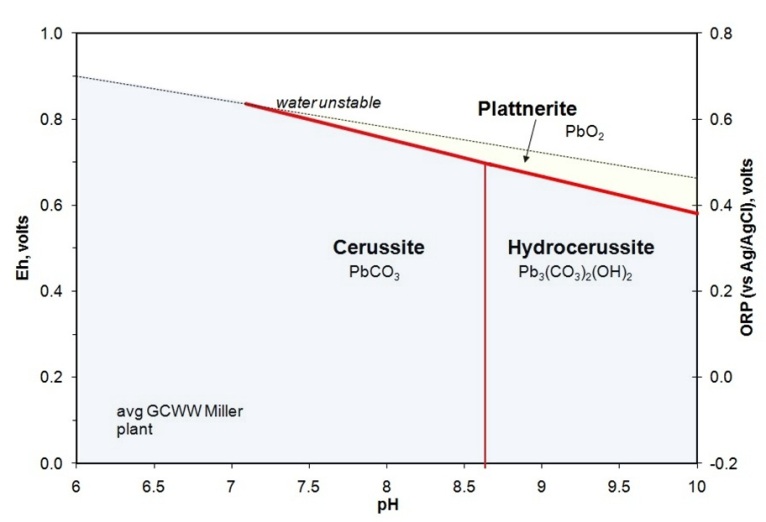
Eh-pH diagram for the Pb carbonates and the oxide plattnerite under alkaline conditions.
If a protective plattnerite layer is present, dissolved Pb levels can be decreased by raising the pH above about 8.5. Note, however, that supersaturation with respect to CaCO3 as calcite occurs in this distribution system at pH values above 8.01. Therefore raising the pH beyond the low 8s is likely to result in an increase in CaCO3 scale formation.

Plattnerite-cerussite relations at very high Eh. The Eh values for this diagram correspond to the upper stability limit for water at a given pH. Waters with free chlorine would have slightly higher Eh (that is water is unstable and treies to react with the chlorine)..
An important aspect of plattnerite chemistry is that the mineral can revert to more soluble Pb phases if the Eh subsequently drops, for example if disinfectant is changed from chlorine to chloramine. Lytle and Schock (2005) presented experimental evidence for ready reversibility of the plattnerite-cerussite transition.
